
Reimbursement for Remedies and Aids
How to get Reimbursement in Germany via Hilfsmittelverzeichnis for Remedies, Medical Aids and assistive Devices?
If you want to successfully sell remedies, medical aids and assistive devices in the German health care market, your medical products would only be reimbursed by German health insurances, if it is listed in the list of Remedies, Medical Aids and assistive Devices, often also referred to as the catalog of medical devices and aids or German Hilfsmittelverzeichnis.
What is the list of Medical Aids or German Hilfsmittelverzeichnis?
The list of medical aids (or German Hilfsmittelverzeichnis), is a systematically structured list of all remedies, medical aids and assistive devices which are reimbursed by the statutory health insurance. It is compiled by the GKV-Spitzenverband in accordance with § 139 SGB V.
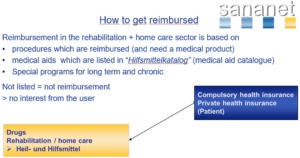
What is Impact of the List of Medical Aids (Hilfsmittelverzeichnis) on your market access to the German Health Care Market?
In principle, the costs of remedies, medical aids and assistive devices in Germany can only be covered by the statutory health insurance if the medical products are listed in the list of medical aids. Although the register of approved aids and appliances not binding in the legal sense, it is very difficult to sell medical remedies and aids that are not listed in the “Hilfsmittelverzeichnis”.
sananet consulting agency could help you to get your remedies, medical aids or assistive devices listed the German Hilfsmittelverzeichnis.
Consulting to get your Remedies, Medical Aids or assistive Devices Reimbursement listed in Germany via Hilfsmittelverzeichnis
sananet consulting agency will be happy to assist you with the listing of your medical products and inclusion in the health insurance schemes “Medical Aids Directory” (German Hilfsmittelverzeichnis) and with changes to their characteristics.
We have already been able to assist many medical device companies and help them with the compilation of documents and clarification of the processes, publications in the directory and compliance with deadlines.
The list of aids is drawn up and continually updated by the GKV (National Association of Statutory Health Insurance Funds).
There is a wide range of medical aids and assistive devices. It includes visual aids, hearing aids, prostheses, orthopedic and other aids ranging from incontinence aids to compression stockings and wheelchairs. Medical aids can also be technical products that are used to administer drugs or other therapeutic agents as e.g. syringes, inhalation devices or application aids.
The medical products and therapeutic appliances that are available in the “Hilfsmittelverzeichns” are allocated to various product groups according to their fields of deployment.
Products are included in the list of aids at the request of the manufacturers (or of third parties empowered by them, like e.g. sananet consulting) if they have specific characteristics and meet certain quality criteria.
How is sananet Consulting Process to get your Medical Aids listed in Hilfsmittelverzeichnis?
Normally, sananet begins projects to include medical aids in the reimbursement catalog with a general analysis of how similar products are currently reimbursed and the best way to secure reimbursement for your medical product. The key question is whether you prefer the simpler and more secure route by using an existing category (if available) with a likely lower price level, or whether you aim to create a new category that better reflects the unique features of your medical device, aid, or equipment.
For the entire reimbursement process, we have a proven procedure structured in several steps: First, we clarify basic information about your company and products. Then, we analyze all relevant products or product groups to determine whether they can be reimbursed under the GKV catalog and in which categories they may fit. Based on this, we develop the individually optimal approach for each product and provide clear recommendations.
The effort required depends on the situation:
-
There is already a code for a comparable competitor product – in this case, the process is straightforward.
-
There is no comparable competitor product, but a suitable category exists – here, the application is somewhat more complex, particularly regarding clinical and health economic data.
-
There is no suitable category or other objective obstacles – then the process may be lengthy, and we explore alternative approaches.
Once the subgroup for your medical aid is defined, the actual application process can begin, including submission or implementation of alternative approaches. In this context, we also support you in marketing and establishing contacts with distributors and strategic partners.
Product subgroups are classified into product types, with associated medical products sharing comparable design features, indications or areas of application, or the same intended purpose and mode of action. Therefore, there is limited flexibility regarding the category in which your medical aid is listed. A useful reference point is always the intended use stated in your CE application.
You can check whether the reimbursement catalog is relevant for your medical product using the following list – or simply contact us. Since the list is generally only available in German, sananet has translated it for you.
| Group | Designation |
| 1 | Suction devices |
| 2 | Adaptation aids |
| 3 | Application aids |
| 4 | Bathing and showering aids |
| 5 | Bandages |
| 6 | Irradiation devices |
| 7 | Aids for the blind |
| 8 | Insoles |
| 9 | Electrostimulation devices |
| 10 | Walking aids |
| 11 | Aids against decubitus |
| 12 | Aids for tracheostoma and laryngectomy |
| 13 | Hearing aids |
| 14 | Inhalation and respiratory therapy devices |
| 15 | Incontinence aids |
| 16 | Communication aids |
| 17 | Aids for compression therapy |
| 18 | Vehicles for the sick/disabled |
| 19 | Nursing articles |
| 20 | Positioning aids |
| 21 | Measuring devices for body conditions/functions |
| 22 | Mobility aids |
| 23 | Orthoses/splints |
| 24 | Leg prostheses |
| 25 | Visual aids |
| 26 | Seating aids |
| 27 | Speech aids |
| 28 | Standing aids |
| 29 | Stoma articles |
| 30 | NN |
| 31 | Shoes |
| 32 | Therapeutic mobility devices |
| 33 | Toilet aids |
| 34 | Hair replacement |
| 35 | Epitheses |
| 36 | Eye prostheses |
| 37 | Breast prostheses |
| 38 | Arm prostheses |
| 50 | Nursing aids to facilitate nursing care |
| 51 | Nursing aids for body care/hygiene and to alleviate complaints |
| 52 | Nursing aids for more independent living/mobility |
| 53 | Nursing aids for the alleviation of complaints |
| 54 | Nursing aids intended for consumption |
| 98 | Other care aids |
| 99 | Miscellaneous |

Contact
info@sananet.com
+49 (0)451 40 08 300

