
Reimbursement of Medical APPs in Germany – DiGA Fast Track – Your Chance for fast Market Access
If you offer a health software as an APP, inclusion in the Directory of Digital Health Applications (DiGA) at the Federal Office for Drugs and Medical Devices (BfArM) is a great opportunity for fast access to the German healthcare market.
Digital health applications (DiGA) can receive provisional reimbursement in Germany via a fast-track application.
Advice on DiGA in Germany
It all seems transparent, and the BfArM has published a guide to the fast-track process that provides an overview of the regulations.
While the application process for digital health apps looks simple enough, there are some pitfalls, especially with timing, that can lead to a negative long-term outcome.
sananet can help you register your Digital Health APP as a Digital Health App, and our guidance can prevent you from ending up with a denial after receiving preliminary reimbursement via a Fast Track application.
What is a DiGA and what are the basic requirements for reimbursement in Germany?
A DiGA is a medical device with the following characteristics:
- A medical device of risk class I or IIa (according to the Medical Devices Ordinance).
- The main function of the digital health app is based on digital technologies, so it is generally a software or health AP
- The medical purpose is essentially achieved through the main digital function
- DiGA supports the detection, monitoring, treatment or mitigation of disease or the detection, treatment, mitigation or compensation of injury or disability
- DiGA is shared by the patient or the healthcare provider and the patient.
Is DiGA Fast Track really fast in Germany?
In principle, yes, because the evaluation of digital health apps follows a fast track to reimbursement and market entry.
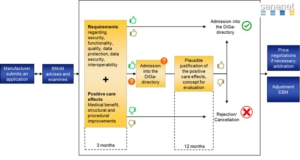
The BfArM must decide whether to approve a digital health app (DiGA) within three months of receiving the manufacturer’s application.
Digital health companies can apply for either final or provisional approval (according to Section 139e (2) and (3) SGB V).
Preliminary listing initiates a 12-month trial period, after which the manufacturer must demonstrate “positive supply effects” (pVE) as the basis for final listing.
The DiGA APP is prescribable and reimbursable in Germany throughout the 12-month trial period. It seems that DiGA fast track with the option of a trial year is a good way to accelerate market entry into the German healthcare market.
However, if you do not use this period prudently and properly, you may end up with a rejection, which will exclude you from the German market permanently or at least for a longer period of time.
sananet advises you on how to make the most of DiGA reimbursement in Germany.
What are the pitfalls in marketing
Once you have provisional or permanent reimbursement for your DiGA, you want to start selling it. However, there are other hidden rules in the German healthcare market that can hinder success. sananet would like to share two specific examples:
Benefits of DiGA
German physicians work in a sectoral reimbursement system. They are not there to perform procedures that benefit other sectors (e.g., hospitals) or reduce overall health care spending. Understanding this system, it becomes clear that the benefit of DiGA must be directly to the prescribing physician.
Since the benefit of the DiGA must be directly to the prescribing physician, either there must be direct payment (which is possible in principle in the DiGA system in Germany) or the physician must work under a selective contract as a DMP or “integrated care,” to name just two examples. A hidden rule is that some DiGAs can reduce the normal remuneration and in this case are not so attractive.
The most important way is to provide a direct benefit to the prescribing physician, as shown above. If this is the case, the physician will recommend it to his patients. An indirect route is patient organizations, which can be helpful. The typical DiGA patient would also search the internet and social media. Advertising is tricky in Germany because there are legal issues.
Privacy security management
DiGAs typically process sensitive personal health data, so data protection is a key concern. For this reason, every new DiGA must be covered by an ISO 27001 information security management system (ISMS) from 2022. But that may not be enough. Germany takes data protection much more seriously than many other countries. A server outside Germany can be a big problem for acceptance, a server outside the EU even a killer for your project.
sananet not only advises you on the fast reimbursement of digital health apps, but also takes care of the successful entry into the German digital health market.

Contact
info@sananet.com
+49 (0)451 40 08 300

