Consulting to get your Medical Software listed as DiPA
Introducton of DiPA in Germany
Digital care applications (DiPA, German for digitale Pflegeanwendung) are applications for mobile devices such as smartphones or tablets as well as web-based applications for laptops or PCs. It can be software or medical devices or a combination. The aim of DiPA is to relieve the burden on carers through digital systems.
In principle, a DiPA can be a native app as well as a desktop or browser application. In addition to the software, a DiPA can also include devices, sensors or other hardware such as wearable sensors or sensors placed in the home environment, as long as the main function is a predominantly digital one.
Now that digital health applications (DiGA) have already provided a relevant impetus for the digitalization of healthcare, the health care software and medical products brought to long-term care as DiPA are now also intended to systematically advance the innovation potential in care in Germany. With the experience sananet consulting agency has with DiGA, we would strongly recommend for health care software and medical product manufacturers to consider to list your life science product as DiPA.
What Impact on your Market Access has it to list your medical Software as DiPA?
In Germany, with the Digital Care and Nursing Modernization Act (DVPMG) on 09.06.2021, digital care applications (digitale Pflegeanwendungen or DiPA) were introduced into outpatient care provision. This means that around 4 million people in need of home care are now entitled to care with DiPA, regardless of their individual care level and regardless of whether the care is provided by their relatives alone or by outpatient nursing and care services. So, if your medical software or product is listed as DiPA, it opens a great opportunity in the German health care market.
How to get your medical Software listed as DiPA in Germany?
sananet consulting agency can help you to get your medical Software listed as DiPA in Germany.
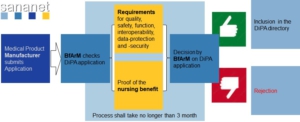
One of the prerequisites for reimbursement by the long-term care insurance funds is that the DiPA has successfully undergone a test procedure at the BfArM and is listed in the so called “Verzeichnis erstattungsfähiger digitaler Pflegeanwendungen (DiPA-Verzeichnis)” or English directory of reimbursable digital care applications (DiPA directory) in accordance with Section 78a (3) of the Eleventh Book of the German Social Code (SGB XI).
Definition of DiPA in Germany
Per definition a digital care applications (digitale Pflegeanwendungen or DiPA) is a health care software or medical products that has the following properties:
- A DiPA may or may not be a medical device. DiPA as medical devices must meet the CE approval in accordance with Regulation (EU) 2017/745 (Medical Device Regulation, MDR) or within the scope of the MDR transitional provisions in accordance with Directive 93/42/EEC Medical Device Directive (MDD) and must belong to low risk class I or IIa.
- The DiPA is essentially based on digital technologies (medical software).
- The nursing benefit is achieved by the DiPA and (if applicable) the eUL required for the DiPA.The DiPA is not a digital application that merely serves to reading or controlling a medical device.
- The purpose of the DiPA is to minimize the impairment of the independence of the person in need of long term medical care or to counteract a worsening of the need for care and thus unfolds its nursing benefits.
- The DiPA can be provided by the person in need of care alone or by the person in need of care in interaction with relatives or nursing services.
- The DiPA can also support family caregivers or other volunteer caregivers in the care of those in need of care.
- The DiPA serves to support those in need of care exclusively in the home context.
What if your Server for DiPA Data Processing outside Germany?
Germany is especially thorough about data privacy. Therefore the permissibility of data processing for medical Software listed as DiPA outside Germany is very strict. BfArM’s reviews procedure pursuant to Section 139e of the Fifth Book of the German Social Code (SGB V). There is a handout on data processing outside Germany (Handreichung zur Datenverarbeitung außerhalb Deutschlands) available which contains up-to-date information.

Contact
info@sananet.com
+49 (0)451 40 08 300

