Consulting on reimbursement of medical devices and medical equipment
sananet consultancy specializes in advising medical device manufacturers on the reimbursement of their medical devices. Through our many years of experience and numerous consulting projects in the area of market introduction of new and innovative medical devices, we know that successful marketing without reimbursement by health insurance companies can be a challenge. However, we would like to emphasize that there are nevertheless ways to realize successful marketing in Germany even without reimbursement, as long as you know how to do it.
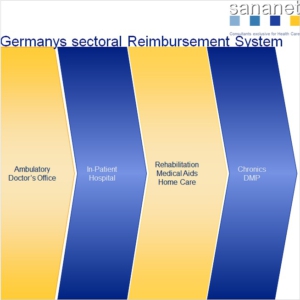
In the following, we offer a brief overview of the reimbursement for medical technology and medical devices in the different reimbursement sectors in Germany.
Reimbursement in the ambulatory sector
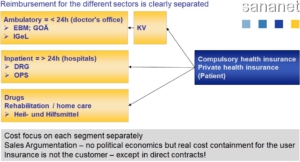
Reimbursement in the outpatient sector is based on the uniform assessment scale (EBM). It is the reimbursement system for contractual medical care in Germany. It is a social insurance-legal register in the German health service, according to which ambulatory and occupancy-medical achievements in the legal health insurance are accounted for.
The German Fee Schedule for Physicians (GOÄ) regulates the billing of medical services outside the scope of contractual medical care in Germany. Private patients, i.e. patients who are insured or uninsured with a private health insurance company and who pay for their treatment themselves, as well as those insured by the statutory health insurance system in the case of so-called individual health services or if they choose the cost reimbursement procedure, receive a private liquidation prepared in accordance with the regulations of the GOÄ.
Learn more about reimbursement in the outpatient sector here.
Reimbursement in the inpatient sector
In the inpatient sector (length of stay in hospital > 24h), the flat-rate DRG system is applied in Germany. This has an extreme impact on cost coverage of your product, since individual procedures and products are usually not reimbursed, but rather a disease-related reimbursement takes place.
To understand the basics of the DRG system, its patient-specific calculation (grouping) and the hospital-internal billing mechanisms are key to market success.
Here you can learn more about reimbursement in the inpatient sector.
Reimbursement for drugs and medical aids
Code number required > new technologies must be listed (basic requirements: CE, study within the EU, observational study).
Payment modalities of the health insurance
a.) Direct reimbursement of prescriptions
b.) Contract
- with pharmaceutical company (discount contract)
- or medical supply store (supply lump sums)
Learn more about the reimbursement of your remedies and aids here.
How to get new Medical Products reimbursed and apply at GB-A
To apply for reimbursement for a new medical product, there is a huge difference where your product is used, in ambulatory or in the in-patient sector. Homecare is again another story.
sananet consultancy here gives a short overview and can assist you in the complicated application process or to find ways to avoid it.
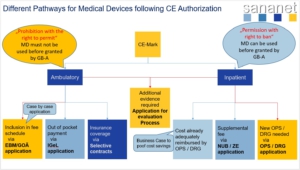
The Federal Joint Committee (G-BA) defines which specific healthcare services are paid for by statutory health insurance. The legal basis for the work of the G-BA is the German Social Code, Book Five (SGB V) The assessment process in Germany is focused on the evaluation of clinical trials
Reimbursement for outpatient and inpatient use of medical devices in Germany differs substantially. After receiving the CE mark, a new diagnostic/treatment method can be sold to a hospital and is reimbursed within existing DRG or OPS as long as it has not been rejected by the G-BA (prohibition right).
Before they can be used, medical devices for outpatient use have to be approved by G-BA based on diagnostic and medical benefit, medical need and efficiency. A new device will be included into the Einheitlicher Bewertungsmaßstab (EBM), i.e., doctor’s fee scale for billing outpatient services in the statutory health insurance. If rejected by G-BA, the MD will not be reimbursed.
Reimbursement for Health Care APPs as DiGA
Under the Digital Healthcare Act (“Gesetz für eine bessere Versorgung durch Digitalisierung und Innovation”), Germany introduced a Digitale Gesundheitsanwendungen (“DiGA”, digital health app) fast track process (the DiGA Fast Track Process) for rapid approval, testing, and reimbursement of digital health apps.
It is a great opportunity for fast access to the German health care market and to get reimbursement for your medical software as an APP. However it is not without pitfalls.
Learn more about the reimbursement of your Health Care APPs as DiGA here.
Reimbursement of costs for digital care applications (DiPA)
In Germany, the Digital Care and Support Modernization Act (DVPMG) introduced digital care applications (DiPA) in outpatient care.
This means that around 4 million people in need of care at home are entitled to DiPA.
Find out more about the reimbursement of your digital care application (DiPA) here

Contact
info@sananet.com
+49 (0)451 40 08 300

